TetraVecta Webinar Recording with XTalks
Lentiviral vectors remain the go-to choice for the efficient delivery of large therapeutic genes to patient cells. In addition to ex vivo indications, the development of lentiviral vectors for in vivo applications is being re-invigorated through more specific targeting. Yet, improvements in both production scale and product quality are needed to realize this. Moreover, the architecture and features of lentiviral vectors used in the clinic have not changed significantly over the last two decades, and so their limitations, such as packaging size limit, or transgene-specific impacts on titer, may impede the success of therapeutic development, therefore increasing the need for 4th generation lentiviral vectors.
This webinar explores Oxford Biomedica’s latest lentiviral vector technology, the TetraVecta™ system, which can significantly enhance the development and manufacturing of safer and more effective lentiviral vector-based therapies. Discover how this advanced technology improves quality, potency and packaging capacity, revolutionizing the manufacturing process. These 4th generation lentiviral vectors have the potential to streamline complex manufacturing processes, enhance productivity and open possibilities for developing new treatments.
The featured speakers review the current state of lentiviral vector engineering, recent breakthroughs, and new ways to make high-titer stable producer cell lines.
This webinar explores Oxford Biomedica’s latest lentiviral vector technology, the TetraVecta™ system, which can significantly enhance the development and manufacturing of safer and more effective lentiviral vector-based therapies. Discover how this advanced technology improves quality, potency and packaging capacity, revolutionizing the manufacturing process. These 4th generation lentiviral vectors have the potential to streamline complex manufacturing processes, enhance productivity and open possibilities for developing new treatments.
The featured speakers review the current state of lentiviral vector engineering, recent breakthroughs, and new ways to make high-titer stable producer cell lines.
Комментарии:
TetraVecta Webinar Recording with XTalks
Oxford Biomedica
your birthday month your dress
Ratika and Harshit
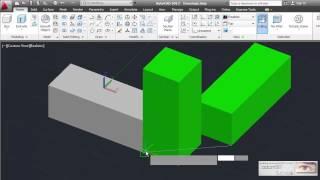
AutoCAD Align objects 3D
adan087 tutorials
Каждый ребенок играя в доктора
Kukla Loli
Eminem НА РУССКОМ
Саша Квашеная
ANGRY MUMMY || Rachit Rojha
Rachit Rojha