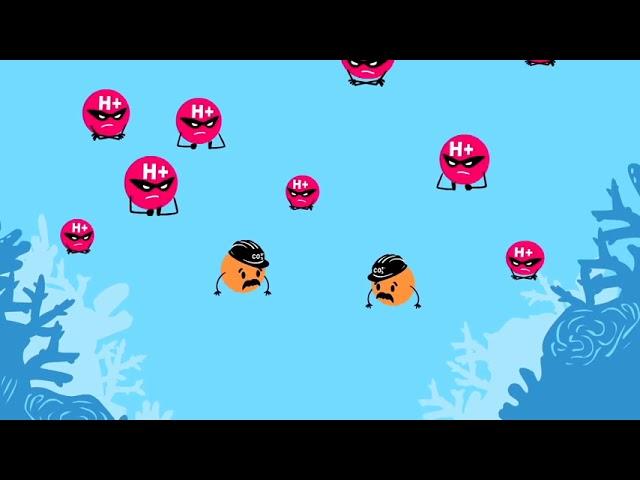
What is Ocean Acidification?
Комментарии:

The fact that factories on land can affect the ocean at all is crazy to think about.
Ответить
This is like the 5th video I’ve had to watch for one assignment and they all say the same thing school is just busy work
Ответить
giggidy giggidy
Ответить
BS
Ответить
YROO XRKSVI! GIRZMTOV!🗿
Surfer girl kinda bad tho i cant lie...

would it help to put organisms in ocean that use carbon dioxide and release oxygen, like some plankton?
Ответить
Plant peepal trees the world's most powerful source of oxygen
Ответить
I love this and who ever made this so much
Ответить
Anyone from jttms 2024 ? 8th grade
Ответить
This video is fire in school
Ответить
Bro I don’t wanna watch this for my science homework 😭
Ответить
The oceans have not become 30% more acidic sine we burnt fossil fuels, that is a lie.
Ответить
Why isn't the H+ formed by CO2 + H2O becoming H3O+? Somebody knows?
Ответить
I edged to this video in class
Ответить
I have read about this in a paper titled "Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms," and that paper, from 2005, suggests that shell degradation will start occurring in 2050, in the southern ocean and then spread globally, if the human carbon footprint stays in a "business as usual" state. The human carbon input to the atmosphere in 2005 was less than 8 gigatons annually. In 2023 the human carbon input is greater than 10 gigatons annually. That is an increase versus static, or "business as usual." My greatest concern is oxygen depletion in the atmosphere due to shell degradation in phytoplankton. Phytoplankton are calcifying organisms that, among other marine organisms, provide a significant amount of the oxygen that we breathe. Phytoplankton are also at the base of the marine food web.
Additionally, any solutions would have to be applied at a similar rate as the pollution is being applied to the atmosphere. For example, applying lye, iron, seaweed, or any other solution would have occur at a similar rate to the pollution that is entering the atmosphere. In 2022, globally, there were 100 million barrels (42 gallons each) of oil consumed each day. Imagine even a fraction of that, 1 million barrels a day, of lye, iron, seaweed, or something else being applied to the ocean, for many years. Should we halt the pollution or should we keep polluting and hope for a technological miracle?

What’s the PH of the ocean ?she forgot to mention it
Ответить
Would dumping lye in the ocean fix it?
Ответить
I hate science class
Ответить
B*ll*cks. As regards ocean acidification, it is estimated that the ocean’s global mean surface pH may have declined (i.e., become less alkaline and thus more “acidic”) by -0.07 to -0.08 in the last 200 years — from pH8.12 during pre-industrial times to 8.04 to 8.05 today (Wei et al, 2015). N.B. The decline in pH occurred before 1930.
However, and very importantly when you look the data after CO2 emissions began rising precipitously in the 1930s, the oceans have become less “acidic”!!!
By way of comparison, from one season to the next, or over the course of less than 6 months, pH levels naturally change by ±0.15 pH units, or twice the overall rate of the last 200 years. On a per-decade scale, the changes are even more pronounced. Oceanic pH values naturally fluctuate up and down by up to 0.6 U within a span of a decade, with an overall range between 7.66 and 8.40. This is decadal rate of pH change is larger than the overall 200-year span (0.07-0.08) by a factor of 8.

Så 1 miljard människor är i beroende av fisk för mat. Nu när enorma mängder av co2 släpps ut så plockar haven upp 50% av all koldioxid. När co2 tar sig in i haven så släpps vätejoner ut. Vätejonerna konkurrerar med marina liv för kolsyran. Vätejonerna plockar upp all kolsyra så att det inte lämnas något för havslivet. Detta gör att marina liv får det svårare att bli stora och dör innan de blivit stora. Det leder till svårigheter för folk att hitta fiskar för att leva. Dessa vätejoner gör även haven surare. Haven har blivit 30% surare sen vi började släppa ut co2 i så stora mängder.
Ответить
Sedimentary Geologist here…
Ocean acidification is the most phenomenal fraud that is being used to scare a scientifically ignorant public.
‘acidification’ is a mere linguistic trick used because acids are scary to the general public.
pH is measured in a logarithmic scale. The average pH of seawater is 8.1. To go from 8.1 to 7.0 (neutral) would require a 110 fold decrease in the amount of carbonate in the water.
Whilst a decrease in pH from 8.1 to 8.0 technically equates to a numerical acidification, to call it acid is an outright lie as a pH of 8.0 is alkaline.
Even if CO2 absorbed from the atmosphere didn’t off gas rapidly, there is so much dissolved carbonate in the earths oceans that it forms limestones beds several kilometres thick.
The chemical buffering capacity of the earths oceans is essentially limitless with an inexhaustible supply of calcium (& magnesium) being supplied by the weathering of minerals at the earth’s surface.
Acidification (and global warming in general) is a colossal fraud.

We need more seaweed
Ответить
no one will ever know i am here that is from my school. whoever sees this and is from my school, bro go pay attention in class. stop looking at this comment.
Ответить
A new "EXISTENTIAL THREAT" manufactured crisis for gung-ho environmentalists to circle the wagons for! 🎪🤡🎪
Ответить
The scientists have not got a clue what they're talking about . Ocean acidification does not exist . The sea is PH 8.1 -- ALKALINE --
Ответить
COMPLETE CRAP! Acidic saltwater? Earthʼs oceans are not acidic and will not become acidic regardless of
the disinformation spread by climate alarmists. Ocean pH has stayed between 7.5 and
8.3 since the first measurements were taken, sometime after 1924 when pH measuring
technology was invented. A pH reading above 7.0 is alkaline, therefore the oceans are
not acidic. It is a false statement to claim, or imply, something is acid when it clearly is alkaline.
What we have here is “climate alarmist science” at work. Itʼs different from real science,
no verification of claims is required. Remember the climate alarmists claims from the 1970s? ʻNuff said.
Earth is a closed system. It was born with all the carbon/carbon dioxide it will ever have
in one form or another. Earth doesnʼt magically acquire CO2 from space and manʼs
influence on CO2 levels in the air or the ocean are inconsequential. Earthʼs soil, water
and air have always contained CO2.
Human blood pH is kept between 7.35 and 7.45 primarily by your lungs and kidneys via
a buffering system. Stabile blood pH is so important that you canʼt change it by eating
an alkaline diet as some have claimed.
Like human blood, seawater is self buffering. If it werenʼt our oceans would have been
made acidic eons ago due to underwater volcanic eruptions and methane vents. Life
would not exist. In fact sea water is 330 times more resistant to pH change than distilled
water and naturally contains 45 times more dissolved CO2 than our atmosphere.
Then thereʼs the Revelle factor. The Revelle factor determines that if the atmospheric
CO2 were doubled (from the current 415 ppm) it would only cause a 10% increase in
oceanic CO2.
When was the last time a climate alarmist mentioned those two last items?
Real science has no need to mislead. Thumbs down on this presentation, itʼs fake
news.

Anyone else here for school?
Ответить
Ocean acidification is a lie the oceans are basic and always will be! They haven't changed in million;s of years! When Co2 is absorbed into water containing Calcium it forms calcium carbonate witch is basic
Ответить
This thing is lame I’m gay
Ответить
your days are numbered, cookycash.
Ответить
The narrator has a nice voice
Ответить
The pH scale is log so every whole number is a power/factor of ten.
By definition pH is the negative exponent of the hydrogen ion concentration.
For instance, pH 9 is 10^-9 or 1 part per billion, 0.000000001.
pH 8 is 10^-8 or 10 parts per billion, 0.000000010.
To go from pH 9 to pH 8 is factor of 10 or 1,000%!!!! Makes 26% look trivial.
Ocean “acidification” of pH 8.2 to pH 8.1 is a decrease in alkalinity equal to 1 ppb of H ions.
I’m fairly certain the ocean flora and fauna don’t even notice.