JEEM 2022 Atoms energy
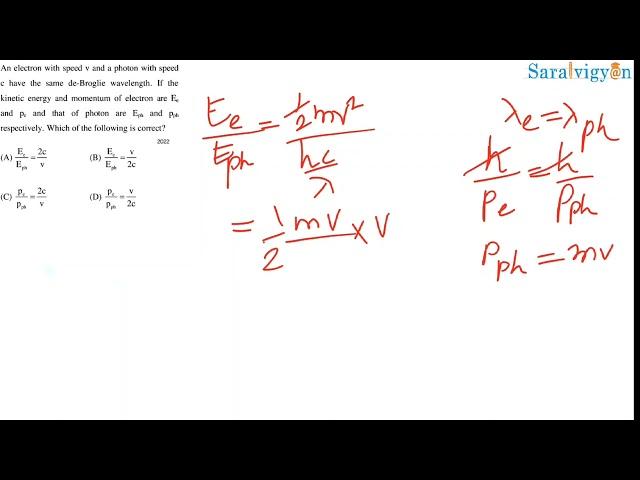
14 An electron with speed v and a photon with speed c have the same de Broglie wavelength. If the kinetic energy and momentum of electron are Ee and pe and that of photon are Eph and pph respectively. Which of the following is correct?
Ee/Eph = 2c/v
Ee/Eph = v/2c
pe/pph = 2c/v
pe/pph = v/2c
de broglie wavelength, momentum
Ee/Eph = 2c/v
Ee/Eph = v/2c
pe/pph = 2c/v
pe/pph = v/2c
de broglie wavelength, momentum
Тэги:
#RUTHERFORD’S_NUCLEAR_MODEL_OF_ATOM #ATOMIC_SPECTRA #Electron_orbits #BOHR_MODEL_OF_THE_HYDROGEN_ATOM #Energy_levels #THE_LINE_SPECTRA_OF_THE_HYDROGEN_ATOM #DE_BROGLIE’S_EXPLANATION_OF_BOHR’S_SECOND_POSTULATE_OF_QUANTISATIONКомментарии:
JEEM 2022 Atoms energy
Physics NEET,JEEMains
Карусель спонсор показа и Рекламный блок 06.05.2023
Ботаник и его друзья
Карусель рекламный блок 11 05 2023
Надежда Исаева
#DailyJonas 002 - Prepare For All Weathers
FILMYARDltd
อยากมีความคล่องตัวไม่ติดขัด ต้องทำอย่างไร?
บารมีหลวงปู่ดู่ พรหมปัญโญ
В Калининградской области 38 туристических проектов получили грантовую поддержку из бюджета
Калининград.Ru — новости Калининграда
[พิเศษ] 50 เรื่องจริง "โลก" ที่คุณอาจไม่เคยรู้ | LUPAS
LUPAS - ลูปัส
JACK HUBER VS ROB MALDONALDO WAR AT TEXAS CLASH 27 "FIGHT OF THE NIGHT" FULL VIDEO
Combat Sports Coverage
Hebrew Anywhere - learning Hebrew online for all levels
Yeda - Education. Moved Forward





![[พิเศษ] 50 เรื่องจริง "โลก" ที่คุณอาจไม่เคยรู้ | LUPAS [พิเศษ] 50 เรื่องจริง "โลก" ที่คุณอาจไม่เคยรู้ | LUPAS](https://rtube.cc/img/upload/RVJMR2RNdzAxWEU.jpg)
![อาจารย์ยอด : มหาเวทย์ป่าช้าทุงมนต์ หลวงปู่หงษ์ พรหมปฺญโญ [พระ] อาจารย์ยอด : มหาเวทย์ป่าช้าทุงมนต์ หลวงปู่หงษ์ พรหมปฺญโญ [พระ]](https://rtube.cc/img/upload/TVJmeTJUdGxPS0M.jpg)