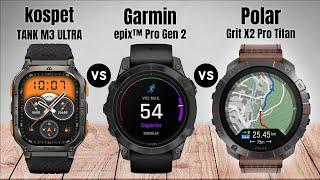
Determine MOLAR SOLUBILITY of Barium sulphate(BaSO4)
This video explains finding molar solubility from solubiltiy product constant.
When sparingly soluble salts like BaSO4 dissolved in water there is the equilibrium between unionised solid and dissociated ions. For example, BaSO4 dissociate into Ba2+ and SO42-.The concentration of these two ions will be molar solubility of BaSO4.
When sparingly soluble salts like BaSO4 dissolved in water there is the equilibrium between unionised solid and dissociated ions. For example, BaSO4 dissociate into Ba2+ and SO42-.The concentration of these two ions will be molar solubility of BaSO4.
Тэги:
#BaSO4_molar_solubility #Ksp_of_BaSO4 #sparingly_solble_salt #solubility_equilibrium #ions #saturated_solution #solublity_product_constant #concentration #equilibrium #molarityКомментарии:
Determine MOLAR SOLUBILITY of Barium sulphate(BaSO4)
Aurora Chemistry
Highlights: Deni Avdija scores 13 in win over Heat
Washington Wizards
Endzeit-News CIA-Agent packt aus! | Erstkontakt mit Aliens im Jahr 2027
EndzeitreporterMcM
КОЗАКИ 3 сильні суперники - яка команда посиплеться перша
ADI - Strategies - Total War!)
WHERE IS GLITCH TECHS SEASON 3 #art #glitchtechs #netflix #trend #short
Nightingale Doodles
Rehman Ali bajwa today's important massage for govt employees and pensioners
Abi Job & Education