Pharma Best Practices Webinars
Master Revised Schedule M – New Critical Requirements for Manufacture of Oral Solid Dosage Forms
Pharma Best Practices Webinars
772
2,573
1 год назад
Understanding Computer System Validation requirements as per revised Schedule M
Pharma Best Practices Webinars
1K
3,895
1 год назад
Understanding Revised Schedule M Part II Ensuring Compliance in Sterile Product Manufacturing
Pharma Best Practices Webinars
1K
4,230
1 год назад
Regulatory Requirements & Expectations for Cleaning & Disinfection of Controlled Manufacturing Areas
Pharma Best Practices Webinars
895
2,984
4 года назад
FDA’s Quality Management Maturity and Quality Ratings Program
Pharma Best Practices Webinars
779
2,598
2 года назад
कैप्सूल कैसे बनते हैं Medicine Capsules Making Process || #shorts #shortsfeed #capsule #pharmcist
PK_Pharma_hub
5
17
1 день назад
Best Practices for responding to FDA 483 observations and Warning Letter
Pharma Best Practices Webinars
1K
4,201
4 года назад
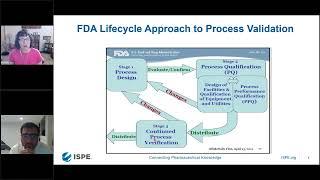
Practical Application Points for Process Validation Lifecycle Approach
Pharma Best Practices Webinars
5K
15,892
5 лет назад
Qualification and Validation principles to meet revised schedule M requirements
Pharma Best Practices Webinars
618
2,059
1 год назад
Points to consider & Line Design for Pre Use Post Sterilization Integrity test
Pharma Best Practices Webinars
2K
5,942
4 года назад
Considerations for Design & Qualification of Single Use Systems
Pharma Best Practices Webinars
679
2,262
4 года назад
Controlling DI Risks With GMP Manufacturing Software
Pharma Best Practices Webinars
359
1,196
4 года назад
GMP Requirements for Pharmaceutical Gases and Clean Compressed Air
Pharma Best Practices Webinars
2K
5,924
3 года назад
Technology Transfer Essentials for Bio Pharmaceuticals
Pharma Best Practices Webinars
756
2,519
4 года назад
Сейчас ищут
Pharma Best Practices Webinars
加拿大留学生移民
𝔽𝕚𝕥𝕠𝕩
영어동요
Хакерские Программы
Drone Dvr
Новоститашкента
Сергей Крикунов
Thinkup Studio Creativo
Sangam Talks
Margdarshan Ias By Siddharth Bhaiya
바른쇼츠
Рыбы Женщина
אורי אלטבוים
Ni Putu Fitri Indriyani
Cool Tech Mukesh
Sami Zhang
W L R
Reasoning Abhishek Kanpur
Mr Velez S Class
Мария Рагуткина
Grc Grg Uhpc Factory Lily
Manjes
Santosh Gawade
음악한줌
Lancelloti
Al Amir الأمير
Creates Diy
Валентина Синчук
Дмитрий Лопухов
영어노래
Acf Mains
Pharma Best Practices Webinars. Смотреть видео: Best Practices For FDA Inspection Readiness, Master Revised Schedule M New Critical Requirements For Manufacture Of Oral Solid Dosage Forms, Understanding Computer System Validation Requirements As Per Revised Schedule M, EMA FDA Expectations In Aseptic Processing.